:quality(80)/business-review.eu/wp-content/uploads/2022/11/unnamed-83.png)
Medical manufacturing is a critical component of the healthcare industry. The products produced by these factories play a vital role in saving lives and improving the quality of life for millions of people every year. To ensure that these products are consistently high quality, manufacturers must adhere to strict quality control procedures.
When most people think about medical manufacturing, they likely think of the assembly-line production of pharmaceuticals and medical devices. However, there is a great deal more to this industry than just the mass production of products. Medical manufacturing encompasses a wide range of activities, from research and development to packaging and sterilization.
This comprehensive guide will provide an overview of the entire medical manufacturing process, from start to finish. So whether you’re looking to enter the industry or simply want to learn more about how it works, read on to find out everything you need to know.
Research and Development
All medical manufacturing begins with research and development. To create new products, manufacturers must first understand the needs of healthcare providers and patients. This requires extensive market research and clinical testing.
The main goal of market research is to identify unmet needs in the healthcare industry. This information is then used to develop new products that can address those needs. To conduct market research, manufacturers typically partner with hospitals, clinics, and other healthcare facilities. These partnerships give manufacturers access to data about the types of products that are in demand and the specific problems that need to be addressed.
Once a manufacturer has a clear understanding of what is needed, they can begin developing prototypes of their products.
CAD and Prototyping
Once a manufacturer has a clear understanding of what their customers want, they can begin developing prototypes of their products. The first step in this process is to create a computer-aided design (CAD) model of the product.
A CAD model is a three-dimensional representation of the product that can be used to create a physical prototype. This model provides manufacturers with a detailed view of the product, as well as its parts. Also, when producing Avery Dennison med 5024 or similar transparent film for electronics, it is important to consider things like thickness and weight, and eventual printing. It can be helpful to use specialized software programs like Autodesk Fusion 360 to create CAD models.
Once the CAD model is complete, the next step is to create a physical prototype of the product. This prototype is used to test the design of the product and to ensure that it meets all of the necessary safety and performance requirements.
Testing
After a prototype has been created, it must undergo a series of tests to ensure that it is safe and effective. These tests are conducted by both the manufacturer and independent third-party organizations.
The first step in testing is to evaluate the safety of the product. This is done by conducting animal studies and clinical trials. Animal studies are used to assess the potential risks associated with using the product. Clinical trials, on the other hand, are used to assess the effectiveness of the product.
After the safety of a product has been established, manufacturers must then obtain approval from regulatory organizations such as the FDA before they can begin mass production. In most cases, manufacturers will need to submit extensive documentation about the product, as well as results from safety and efficacy trials, before they can obtain approval.
Mass Production
Once a product has been approved for mass production, the manufacturing process can begin. In most cases, medical products are produced using automated assembly lines. This allows manufacturers to produce large quantities of products quickly and efficiently.
During the production process, manufacturers must adhere to strict quality control procedures to ensure that their products meet all safety and performance standards. Once the products have been manufactured, they must undergo final testing before they can be shipped to customers.
Packaging and Sterilization
After a product has been produced, it must be properly packaged and sterilized before it can be used. Packaging is important because it helps to protect the product from damage during shipping and storage. In most cases, medical products are packaged in sterile containers that prevent bacteria and other contaminants from coming into contact with the product.
Sterilization is another important step in the manufacturing process. This is done to kill any bacteria or other microorganisms that may be present in the product. Several different methods can be used to sterilize medical products, such as heat, radiation, and chemicals. Depending on the type of product, one or more of these methods may be used. Products that are sterile and properly packaged can then be shipped to customers.
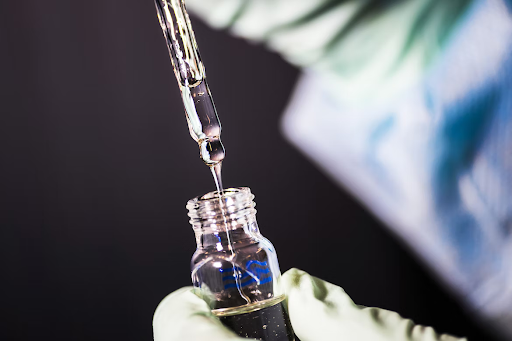
The manufacturing process of medical products is complex and regulated. In most cases, it takes several years for a product to go from concept to mass production. However, the result is a safe and effective product that can improve the lives of patients. As long as manufacturers continue to adhere to strict safety and quality standards, medical products will continue to be a vital part of the healthcare industry.



:quality(80)/business-review.eu/wp-content/uploads/2024/07/vodafone-RO.jpg)



:quality(80)/business-review.eu/wp-content/uploads/2024/06/22C0420_006.jpg)

:quality(80)/business-review.eu/wp-content/uploads/2024/06/COVER-1-4.jpg)



:quality(80)/business-review.eu/wp-content/uploads/2024/06/br-june-2.jpg)
:quality(50)/business-review.eu/wp-content/uploads/2024/07/BeFunky-collage-37-scaled.jpg)
:quality(50)/business-review.eu/wp-content/uploads/2024/07/04_ThinkPad_T14s_6_Business_Coworking.jpg)
:quality(50)/business-review.eu/wp-content/uploads/2024/07/Iulia-Surugiu-scaled.jpg)